The Kühn and Liu Lab within the Weill Cornell medicine conducts cutting-edge investigations into the cellular and molecular mechanisms of the heart muscle in search of a cure for heart failure. The long-term objective is to provide cellular and molecular targets for therapeutic treatment of heart failure and congenital heart disease.
We know that natural cardiomyocyte proliferation is not sufficient to regenerate heart muscle defects in babies and children with congenital heart disease or in adults after a heart attack. Heart failure can be the result.
By focusing on the mechanisms of growth and regeneration of the myocardium, Dr. Kühn and Dr. Liu’s research has already led to important discoveries. Their work has shown that administration of two naturally occurring peptides, periostin and neuregulin, can stimulate cardiomyocytes to proliferate and, in animals, repair myocardial defects and restore cardiac function.
Members of the Kühn and Liu Lab team are focused on three initial areas
- Inducing Cardiomyocyte Proliferation
- Mechanisms of Myocardial Growth and Regeneration
- Translational Research
The Kühn and Liu Lab team are taking these findings and turning them into new and novel therapies for healing the hearts of children and adults.
1. Inducing Cardiomyocyte Proliferation
The Kühn and Liu Lab discovered that cell cycle re-entry and division of cardiomyocytes is induced by periostin peptide, a component of the extracellular matrix, and neuregulin 1 (NRG1), a growth factor. After experimentally inducing myocardial infarctions, we have shown that the administration of recombinant periostin peptide or NGR1 enhances cardiomyocyte cycling, reduces infarct size, and improves myocardial function. Using an animal model to mimic heart failure in babies and children, we are currently testing the therapeutic benefits of NGR1 administration.
We have demonstrated that a sub-population of differentiated cardiomyocytes has proliferative potential and responds to periostin peptide and NGR1 with cell cycle re-entry.
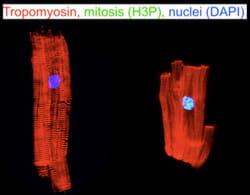
2. Mechanisms of Myocardial Growth and Regeneration
Regeneration is an important mechanism of tissue homeostasis in multicellular organisms, and the limited ability of the mammalian heart to regenerate is remarkable. Many mammalian tissues, such as blood and skin, regenerate after injury, relying on undifferentiated stem cells.
Heart tissue, like other tissues with limited regenerative capacity, is largely comprised of terminally differentiated cells. This means that they do not divide. However, some cardiomyocytes can re-enter the cell division cycle under certain conditions. For example, cardiomyocyte cell cycle activity in the region bordering a myocardial infarction increases transiently. Although this increase is not sufficient for effective regeneration, it suggests that some cardiomyocytes may proliferate in response to extracellular signals present in the infarct border zone.
The Kühn and Liu Lab is exploring the signals that promote cardiomyocyte cell cycle entry and division with the hope of identifying specific factors that can become leads for new medications
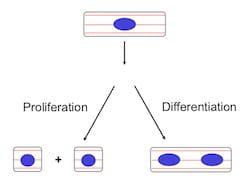
3. Translational Research
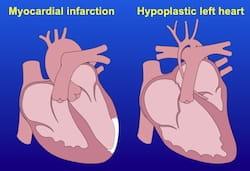
Stimulating cardiomyocyte proliferation with periostin peptide or with neuregulin 1 shows great potential for repairing the mammalian heart after injury due to myocardial infarction, or to repair congenital heart defects, such as hypoplastic left heart syndrome.
As a step toward this goal, researchers in the Kühn and Liu Lab are currently working to characterize cardiomyocyte proliferation in children and adults with heart failure.
We have demonstrated that cardiomyocyte proliferation contributes to heart growth in babies and children. This raises the possibility to target this process with new regenerative therapies. One of our regeneration factors, neuregulin, is currently in clinical phase 2 testing in adult heart failure patients. We do not have financial interests in the development of neuregulin as a therapy and look forward to advancing toward clinical trials in children. The significance and promise of research on cardiomyocyte regeneration for developing new heart failure therapies for babies and infants was recognized by a working group of the National Heart Lung and Blood Institute in 2013.